ONGOING RESEARCH
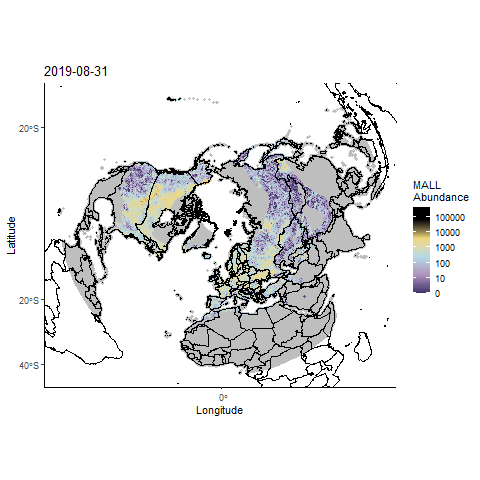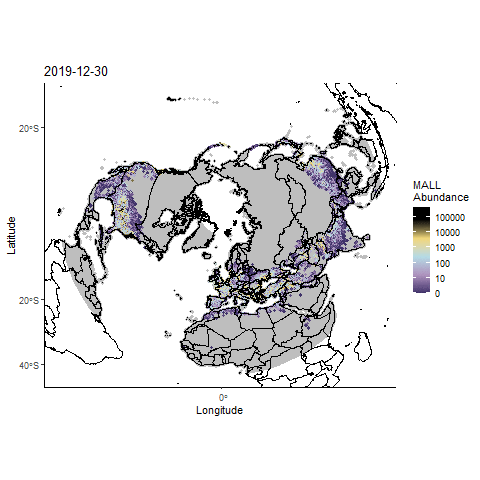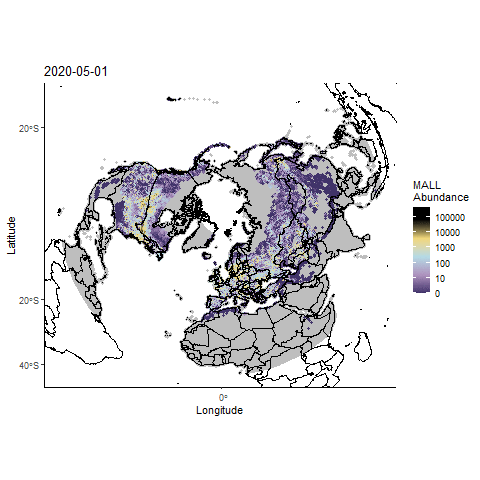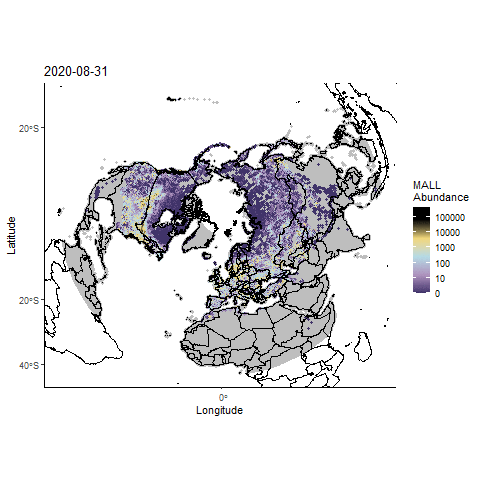
Forecasting highly pathogenic avian influenza dispersal in migrating waterfowl and spillover risk for domestic poultry
|

Host-environment interactions and survival of bats with white-nose syndrome
The way hosts use their environment can greatly influence outcomes of disease. Temperature and humidity microclimates that would be enticing under normal circumstances may become ecological traps when a pathogen that thrives in those microclimates is introduced. This is one hypothesis for why many bats have died of white-nose syndrome in North America: the conditions that susceptible bats use to hibernate overlaps heavily with the conditions in which the pathogenic fungus Pseudogymnoascus destructans thrives. I am examining how bats of heavily-affected species can use their environment to survive disease while other regional populations experience extreme mortality. Using individual morphometrics and microclimate data gathered by novel bat-fit miniaturized data loggers, I am developing Bayesian hierarchical models that predict individual bat hibernation energy use and survival with white-nose.
The way hosts use their environment can greatly influence outcomes of disease. Temperature and humidity microclimates that would be enticing under normal circumstances may become ecological traps when a pathogen that thrives in those microclimates is introduced. This is one hypothesis for why many bats have died of white-nose syndrome in North America: the conditions that susceptible bats use to hibernate overlaps heavily with the conditions in which the pathogenic fungus Pseudogymnoascus destructans thrives. I am examining how bats of heavily-affected species can use their environment to survive disease while other regional populations experience extreme mortality. Using individual morphometrics and microclimate data gathered by novel bat-fit miniaturized data loggers, I am developing Bayesian hierarchical models that predict individual bat hibernation energy use and survival with white-nose.
PREVIOUS PROJECTS

Predicting conditions that promote evolutionary rescue in host-pathogen systems
Novel pathogen introduction can have drastic consequences for naive host populations, and outcomes can be difficult to predict. Evolutionary rescue (ER) provides a foundation for understanding whether hosts are driven to extinction or survive via adaptation. Currently, patterns of host population dynamics alongside evidence of adaptation are used to infer ER. However, the gap between established ER theory and complexity inherent in natural systems makes interpretation difficult because these patterns can be confounded with ecological drivers of survival under the current theory. To bridge this gap, we expanded ER theory to include biological selective agents and developed a novel framework for evaluation of ER potential within natural systems to identify system characteristics that make ER possible. We use the plague system of Yersinia pestis infecting black-tailed prairie dogs and California ground squirrels as a case study.
Novel pathogen introduction can have drastic consequences for naive host populations, and outcomes can be difficult to predict. Evolutionary rescue (ER) provides a foundation for understanding whether hosts are driven to extinction or survive via adaptation. Currently, patterns of host population dynamics alongside evidence of adaptation are used to infer ER. However, the gap between established ER theory and complexity inherent in natural systems makes interpretation difficult because these patterns can be confounded with ecological drivers of survival under the current theory. To bridge this gap, we expanded ER theory to include biological selective agents and developed a novel framework for evaluation of ER potential within natural systems to identify system characteristics that make ER possible. We use the plague system of Yersinia pestis infecting black-tailed prairie dogs and California ground squirrels as a case study.